Morphology
The morphology of Clibanarius longitarus and other hermit crabs is highly specialised for their unique niche of living inside empty gastropod shells. It is believed that ancestors of hermit crabs occupied small cracks and crevasses in rocks for protection, and later developed the ability to occupy shells to increase mobility, (Ruppert, Fox & Barnes, 2004.) Today, hermit crab's bodies have evolved to fit into right handed or dextral, discarded snail shells, (Ruppert, Fox & Barnes, 2004; Tudge, Asakura & Ahyong, 2004.) Although there are rarer cases of hermit crabs occupying left-handed shells or appropriately sized pieces of human trash, (See Figure 3.) The Blue striped hermit crab, like all other hermit crabs, need to increase the size of their shell as they grow larger. When an appropriate new shell is found, the transition into it is fast, as the crab is very vulnerable to predation with an exposed, unprotected, abdomen. As they are dependant on empty gastropod shells, the number of available shells may limit local populations, (Ruppert, Fox and Barnes, 2004.)

Figure 3- A terrestrial hermit crab species utilising a piece of rubbish as a shell, (Source: Carter K. 2010)
Males are larger than females, (Litulo, 2005.) This if found to be common among several other species of tropical hermit crab, (turra & Leite, 2000; Branco et al., 2002; Martinelli, 2002.) This sexual dimorphism occurs because of one, more, or all of the following three possible reasons. Males may grow larger because they can direct more energy into growth and metabolism and not in brooding offspring. A second possible cause of this dimorphism is that males have evolved to be larger to better their chance of winning intraspecific fights for females. Finally, larger males might have evolved as a result of having more reproductive potential and having a better chance of copulating with numerous females (Abrams, 1988.) Hermit crab growth may also correlate with seasons in certain locations. A study by Bertness, (1980), found that hermit crab growth rates increased during the dry season of the Panama canal because of the the nutrient increase from oceanic upwelling.
The colouration of the Blue striped hermit crab can vary substantialy depending on global location and habitat. An example of this was found in an investigation done in the mangrove habitats of Papua, Indonesia, where the blue stripe along the pereiopods was far more vibrant in species living in dense mangrove forests, (Rahayu, 2003.)
The carapace is somewhat flat, and is only calcified at the anterior end. This region is also referred to as the shield. The abdomen, or properly termed pleon, is soft, membranous, and twisted dextrally to fit into shells. Apart from having a shell, one of the most distinguishing features of hermit crabs is that their body plans are asymmetrical, not only by having two different size chelipeds (a characteristic which many decapods have) but in the abdomen too, (Tudge, Asakura & Ahyong, 2004.)
Like all crustaceans, hermit crabs have biramous appendages. Clibanarius longitarus have two pairs of antennae, the smaller of the two properly termed the antennulae. Each have one or two multi-segmented flagella arising from a peduncle. The antennae have only one flagellum but it is the longest. These flagellum bear a line of aesthetascs, or chemosensory structures. The mandibles are the inner most mouth parts of the buccal frame, and are well developed in this species. Their palps have three segments, the last being equipped with setae along the margin. The maxillule is the second mouth part appendage, followed by the maxilla, which is well developed and lobe shaped. In the superfamily paguroidea the maxilla is used to create a respiratory current. For this reason they are heavily equipped with setae. The first maxilliped, or fourth mouth appendage, is shaped wider close to its base and narrow towards the end. The endopod, or inner branch of the biramous appendage, is small and bears a multiarticulate flagella. The second and third pairs of maxillipeds have a much longer multiarticulate flagellum, and have a much more developed endopod, (Tudge, Asakura & Ahyong, 2004.)
The Blue striped hermit crab has five pairs of legs, or pereiopods, (see Figure 6.) The first are the largest and most robust, for they are the claws, or chelipeds. Cheliped size differences are common among decapod species, so it is not uncommon to see the right claw being larger than the left as seen in the Blue striped hermit crab, (see Figure 4.) Interestingly, this particular species belongs to the family diogenidae, which essentially means 'left-handed', and most members of this group have a larger left cheliped than their right, with a few exceptions. Clibanarius longitarus is one of those exceptions. Hermit crabs use their chelipeds not only for grasping things but also as an operculum for protection when they are retracted into their shell.

Figure 4- Clibanarius longitarus have a larger right cheliped, (Source: Author, 2014)
The second and third pereiopods are the crab's walking legs, they are the longest appendages of Clibanarius longitarus but slightly less robust than the chelipeds, (see Figure 6.) These pereiopods carry the characteristic blue stripe seen in this species, (see Figure 5.)

Figure 5- The blue stripes down the second and third pereiopds can be seen clearly here, (Source: Wild Singapore, 2008)
The fourth and fifth pereiopods are reduced in this species, largely due to the fact that they must fit into the shell, (see Figure 6.) They are morphed in a way that allows them to brace themselves and control their position within the shell. These pereiopods are able to operate rapidly when required and are able to pull the crab back into its shell at great speeds. This is necessary when being attacked by a predator or another crab who may desire its shell. For this function, these pereiopods are armed with corneous spines or teeth to increase grip on the insides of the shell.
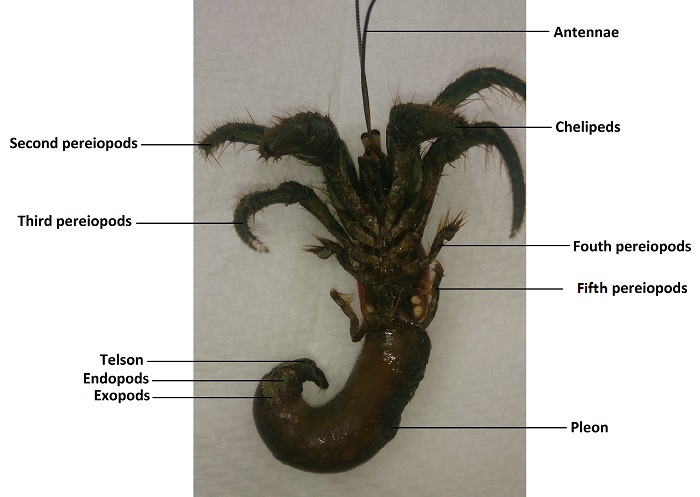
Figure 6- Annotated photograph of Clibanarius longitarus showing some major appendages, (Source: Author, 2014)
Pleopods are small paired appendages that are located in segments posterior to the pereiopods. In all hermit crabs, they are reduced for the purpose of fitting inside the deeper areas of the shell cavity. In the Clibanarius genus, the first pair of pleopods, and all pleopods on the right side of the body have been lost completely. The number of pleopods on the left hand size can differ between males and females; females usually have their second, third, fourth, and fifth appendages present, where as males can sometimes be missing only their second pleopod, (see Figure 7.)

Figure 7- The final pleopod of Clibanarius longitarus, (Source: Author, 2014)
The telson is the most posterior segment of the body. Unlike the rest of the abdomen, it is calcified like the carapace. It also bears uropods, paired appendages more specifically known as endopods and exopods. The entrance to the anus is located in this final segment, (see Figure 6; Figure 8.)

Figure 8- The telson of Clibanarius longitarus, (Source: Author, 2014) |